Arrows in the energy diagram below represent enthalpy changes occurring in the exothermic formation of a solution:
ΔHsoln = enthalpy of solution
ΔHsolute-solute = enthalpy change involving solute-solute interactions
ΔHsolute-solvent = enthalpy change involving solute-solvent interactions
ΔHsolvent-solvent = enthalpy change involving solvent-solvent interactions 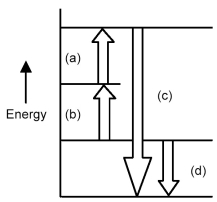
-Which arrows represent ΔHsolute-solute and ΔHsolvent-solvent?
Definitions:
Refreshments
Light snacks and drinks provided to revitalize energy and concentration, often during meetings or public gatherings.
Technical group
A team comprised of individuals with specialized skills and knowledge focused on specific technical areas or tasks.
Top management
The highest level of managerial roles in an organization, responsible for setting strategic goals and making overarching decisions.
Managerial
Pertaining to the duties and activities involved in managing an organization or part of it.
Q21: The HI bond has a length of
Q31: A solution is prepared by dissolving 40.0
Q42: The reaction below is second order in
Q67: A 2.00 M solution of CaCl<sub>2</sub> in
Q116: Which of the following Br∅nsted-Lowry acids does
Q149: If the total pressure in the container
Q151: An unknown gas effuses 1.73 times faster
Q184: Which statement is true for the general
Q195: Consider the first-order decomposition of A molecules
Q205: Calculate the hydronium ion concentration in an