The following diagram shows a close-up view of the vapor pressure curves for a pure solvent and a solution containing a nonvolatile solute dissolved in this solvent. 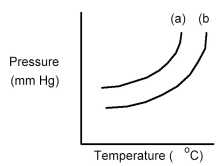
-Which curve is the solvent and what happens to the boiling point when the solute is dissolved in the solvent?
Definitions:
Triacylglycerol
The main storage lipid of organisms, consisting of a glycerol combined chemically with three fatty acids; also called triglyceride. Compare with monoacylglycerol and diacylglycerol.
Nucleic Acid
Nucleic acids are biopolymers, or large biomolecules, essential for all known forms of life, including DNA and RNA, which carry genetic information.
Alpha-Helical Structure
A common motif in the secondary structure of proteins, characterized by a right-handed coil that provides structural support.
Hydrogen Bonds
Weak bonds formed between a hydrogen atom covalently bonded to one electronegative atom and another electronegative atom, playing a critical role in the structure of water and biological molecules.
Q23: Ethyl chloride,C<sub>2</sub>H<sub>5</sub>Cl,is used as a local anesthetic.It
Q90: What is the weight percent of a
Q91: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298
Q109: An aqueous CsCl solution is 8.00 wt%
Q117: What is the pressure in a gas
Q129: Given the hypothetical reaction: 2 A(s)+ x
Q139: The following reaction is second order in
Q153: In the process of dissolving ionic compounds,the
Q176: The layers of graphite are held together
Q189: For the first-order reaction,2 N<sub>2</sub>O(g)→ 2 N<sub>2</sub>(g)+