A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 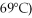 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 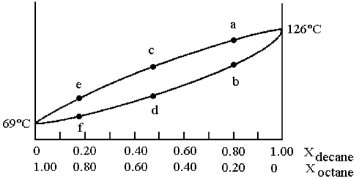
-Assume that the vapor at point c is condensed and reboiled.What is the boiling point?
Definitions:
Ernest Becker
An American anthropologist and writer known for his works on death and the meaning of life, notably "The Denial of Death."
Adolescence
A developmental stage between childhood and adulthood, characterized by physical, psychological, and social changes.
Coping with Demands
The strategies or mechanisms that individuals use to manage the stress and challenges presented by life's demands and expectations.
Intimate Relationships
Close, personal connections between individuals, characterized by affection, love, trust, and commitment.
Q34: Which general rate law below corresponds to
Q103: The decomposition of dinitrogen pentoxide is described
Q136: What is the physical phase of the
Q143: In liquid propanol,CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>OH,<sub> </sub>which intermolecular forces are
Q149: If the total pressure in the container
Q155: A gaseous compound,C,undergoes catalytic decomposition at an
Q157: When dissolved in water,which of the following
Q168: Over the time interval 300 to 400
Q179: Fluorine-18 is a radioisotope widely used in
Q180: In which case should CO<sub>2</sub>(g)be more soluble