Consider the first-order reaction A → B in which A molecules (shaded spheres) are converted to B molecules (unshaded spheres) . 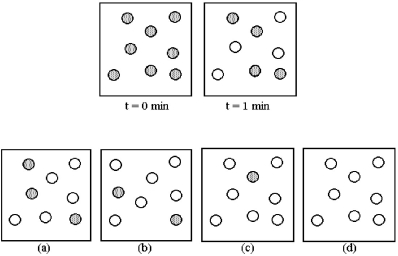
-Which drawing (a) -(d) represents the reaction mixture at t = 2 minutes?
Definitions:
Z Score
A statistical measurement that describes a value's relationship to the mean of a group of values, measured in terms of standard deviations from the mean.
Mean
The mean is a measure of central tendency that calculates the average value of a set of numbers by dividing the sum of those numbers by their quantity.
Z Score
A statistical measure that describes a value's relationship to the mean of a group of values, measured in terms of standard deviations from the mean.
Scores
Quantitative values assigned to individuals to represent or measure specific traits or outcomes.
Q2: Which of the following mixtures have components
Q4: If figure (1)represents the vapor pressure of
Q48: The Henry's Law constant of methyl bromide,CH<sub>3</sub>Br,is
Q105: The change in the Gibbs free energy
Q111: The coordination number of each atom in
Q165: What is the physical phase of the
Q166: For the reaction shown below,which change in
Q176: Consider the first-order reaction A → B
Q187: At a given temperature the vapor pressures
Q202: From the following chemical reactions determine the