Consider a reaction that occurs by the following one-step mechanism:
A2 + B2 → 2 AB
The potential energy profile for this reaction is shown below. 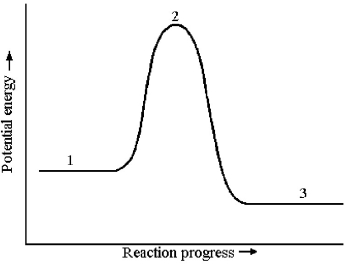
-The activation energy for the forward reaction is given by the difference in energy between which two reaction stages?
Definitions:
Economically Successful
A state of achieving significant financial prosperity or wealth, often linked to effective management of resources and investments.
Educationally Successful
Achieving significant accomplishments or obtaining high levels of performance in educational settings, often measured by grades, graduation, or other academic achievements.
Acculturation
The process of adopting the cultural traits or social patterns of another group, especially by contact and exchange.
Assessment
The process of evaluating or analyzing something to determine its nature, quality, or significance.
Q23: Which of the following solutions will have
Q50: The reaction for the decomposition of
Q111: The coordination number of each atom in
Q124: What is the activation energy for the
Q131: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4939/.jpg" alt=" has a
Q173: In the drawing of acetaldehyde,CH<sub>3</sub>CHO,the largest partial
Q178: Which of these neutralization reactions has a
Q197: Erythromycin is a basic antimicrobial with pK<sub>b</sub>
Q200: The reaction: 2 HI → H<sub>2</sub> +
Q207: Which one of the following is expected