Consider a reaction that occurs by the following one-step mechanism:
A2 + B2 → 2 AB
The potential energy profile for this reaction is shown below. 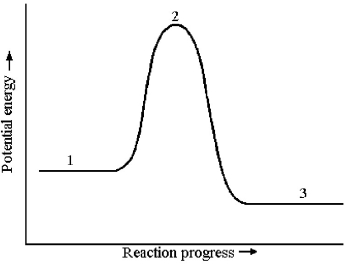
-The energy of reaction,ΔE,is given by the difference in energy between which two reaction stages?
Definitions:
Sensory Systems
The parts of the nervous system that detect changes in the environment and transmit this information to the brain.
Supertaster
An individual who experiences taste with far greater intensity than the average person, due to having more taste buds.
Door-In-The-Face Technique
A persuasion strategy where a larger, unreasonable request is made first and is expected to be refused, only to be followed by a smaller, more reasonable request.
Olfactory Cilia
Hair-like structures on the olfactory receptor neurons within the nose that serve as sites for odorant molecules to bind, initiating the perception of smell.
Q9: Nitrogen dioxide decomposes at 300°C via a
Q17: The vapor pressure of water at 25°C
Q62: Rhodium has a face-centered cubic structure and
Q79: Which type of spherical packing has the
Q101: While mercury is very useful in barometers,mercury
Q104: Which is expected to have the largest
Q125: What is the overall reaction order for
Q134: Arrange the following 0.10 M aqueous solutions
Q170: A mechanism for a naturally occurring reaction
Q200: Calculate the pH of a 0.100 M