Shown below is a concentration vs.time plot for the reaction A ⇌ B.For this reaction the value of the equilibrium constant is 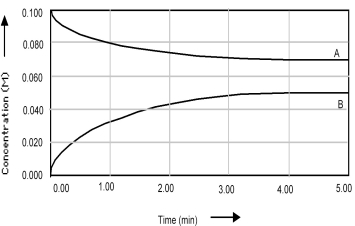
Definitions:
Involuntary Bankruptcy
This is a legal process initiated by creditors against a debtor, rather than the debtor filing for bankruptcy voluntarily, forcing them into bankruptcy to satisfy debts.
Creditors
Persons or organizations that are owed money by borrowers.
Bankruptcy Proceedings
The legal process through which individuals or businesses that are unable to repay their debts can seek relief from some or all of their financial obligations.
Proof of Claim
A written statement filed by a creditor in bankruptcy proceedings detailing the claim against the debtor.
Q25: For which solution(s)is pH = pK<sub>a</sub>?<br>A)only solution
Q38: The following pictures represent mixtures of A<sub>2</sub>B<sub>4</sub>
Q41: If solution (1)is a saturated solution of
Q70: A solution is prepared by dissolving 171
Q97: What is the pH of a buffer
Q124: What is the activation energy for the
Q140: For the reaction shown below,which change in
Q142: State whether the solubility of Cu(OH)<sub>2</sub> will
Q164: What volume of 3.00 M CH<sub>3</sub>OH solution
Q209: A Br∅nsted-Lowry acid is best defined as