The following pictures represent mixtures of cis-C2H2X2 molecules and trans-C2H2X2 molecules,which interconvert according to the equation cis-C2H2X2 ⇌ trans-C2H2X2.If mixture (1) is at equilibrium,which of the other mixtures are also at equilibrium? 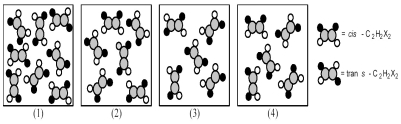
Definitions:
Q6: A solution with a hydroxide ion concentration
Q19: What is true about the relationship of
Q20: Sodium hydroxide is available commercially as a
Q67: What is the K<sub>a</sub> of the amino
Q84: Which drawing (a)-(d)represents the reaction mixture at
Q111: Calculate the molar solubility of thallium(I)chloride in
Q120: Which metal sulfides can be precipitated from
Q168: What is the molar solubility of AgCl
Q187: At a given temperature the vapor pressures
Q195: Consider the first-order decomposition of A molecules