The following picture represents the equilibrium state for the reaction A2 + B2 ⇌ 2AB.What is the relationship between the rate constant for the forward reaction,kf,and the rate constant for the reverse reaction kr? 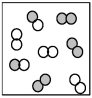
Definitions:
Test Scores
Numerical or grading assessments that evaluate a person's performance on academic or skill-based tests.
Aptitude Tests
Standardized tests designed to measure an individual's potential to succeed in a particular activity or field of study.
Significance Level
The threshold below which a p-value is considered statistically significant, typically set at 0.05 or 5%.
Population Means
The average value of a particular characteristic for all the individuals within a population.
Q36: Calculate the pH of a 0.020 M
Q67: A researcher needs 5.00 mg of <sup>128</sup>Ba
Q71: Naproxen is a commercially important anti-inflammatory agent
Q76: At 25°C the vapor pressures of benzene
Q97: What is the pH of a buffer
Q111: If the units for rate are M
Q135: Identify the Br∅nsted-Lowry acids.<br>A)(1)and (3)<br>B)(1)and (4)<br>C)(2)and (3)<br>D)(2)and
Q146: A crude type of disappearing ink is
Q163: CaF<sub>2</sub> has K<sub>sp</sub> = 3.5 × 10<sup>-11</sup>.If
Q206: Fluorine-18 is an isotope used in Positron