The reaction A2 + B2 ⇌ 2AB has an equilibrium constant Kc = 1.8.The following pictures represent reaction mixtures that contain A2 molecules (shaded) and B2 molecules (unshaded) ,and AB molecules. 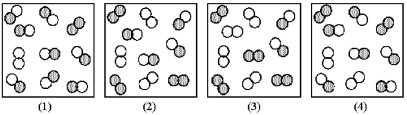
-Which nonequilibrium mixture will react in the forward direction to reach equilibrium?
Definitions:
Time-management Pitfalls
Common mistakes or problems that can disrupt effective time management and planning.
Social Aspects
The elements of life that involve interaction and relationships with other people within a society.
Weekly Planner
A tool or document used to organize and schedule tasks, appointments, and goals over the course of a week.
Study Time
A designated period devoted to academic learning or review.
Q32: How many grams of pyridine are there
Q38: The balanced net ionic equation for the
Q56: For the reaction A<sub>2</sub> + 2 B<sub>3</sub>
Q60: Which picture (2)-(4)represents the equilibrium mixture when
Q73: What is the second stepwise equilibrium constant
Q74: What is the hydronium ion concentration in
Q153: Molecular hydrogen can be made from methane
Q170: A mechanism for a naturally occurring reaction
Q171: Using the method of initial rates for
Q179: Potassium chromate is slowly added to a