Picture (1) represents the equilibrium mixture for the reaction A + B ⇌ 2 AB at 298 K. 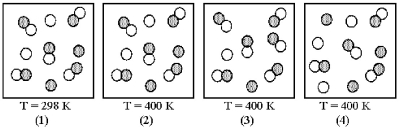
-If this reaction is exothermic,which picture (2) -(4) represents the equilibrium mixture at 400 K?
Definitions:
Standard Deviations
A metric indicating the degree of spread or variability in a collection of numbers, highlighting the distance of individual values from the central mean.
Chebyshev's Theorem
A statistical theorem which states that for any real number greater than one, a certain proportion of values in any data set will be close to the mean, based on the number of standard deviations specified.
Standard Deviations
A measure of the amount of variation or dispersion of a set of values, indicating how spread out the values are from their mean.
Midrange
The arithmetic average of the maximum and minimum values in a data set.
Q11: Which of the following is not an
Q14: What is the hydronium ion concentration of
Q54: Given the reaction at a certain temperature:
Q61: What is the approximate pH at the
Q74: What is the hydronium ion concentration in
Q87: For the reaction shown below the value
Q113: Predict the sign of ΔS of the
Q115: The equivalence point pH of the titration
Q157: Which point a-d represents a buffer region?<br>A)point
Q197: At an elevated temperature the decomposition of