What is the molar solubility of AgCl in 0.10 M NaCN if the colorless complex ion Ag(CN) 2- forms? Ksp for AgCl is 1.8 × 10-10 and Kf for Ag(CN) 2- is 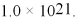
Definitions:
Payoffs
The returns or outcomes from different decisions or actions in decision theory and economics.
Expected Monetary Values
A calculation used in decision-making to determine the probable monetary gain or loss from an action, by multiplying possible outcomes by their respective probabilities and summing these products.
Payoffs
The returns or outcomes received from a particular action or investment, often discussed in game theory and economics.
Decision Alternative
Different courses of action or strategies that a decision-maker can choose from in a decision-making process.
Q7: What is the pH of a 0.30
Q11: During perspiration,<br>A)the entropy of the water evaporated
Q25: How many mL of O<sub>2</sub> gas at
Q33: Which of the following salts are acidic?<br>A)LiCl,NaCl,KCl<br>B)NH<sub>4</sub>Cl,CuCl<sub>2</sub>,AlCl<sub>3</sub><br>C)NaCH<sub>3</sub>CO<sub>2</sub>,KCH<sub>3</sub>CO<sub>2</sub>,RbCH<sub>3</sub>CO<sub>2</sub><br>D)NaCl,NH<sub>4</sub>Cl,Na<sub>2</sub>CO<sub>3</sub>
Q44: The equivalence point pH of the titration
Q85: TRIS {(HOCH<sub>2</sub>)<sub>3</sub>CNH<sub>2</sub>} is one of the most
Q98: Phosphorus pentachloride decomposes to phosphorus trichloride at
Q104: If ΔG° is negative for a reaction,<br>A)K
Q136: What is the hydronium ion concentration of
Q173: What is the strongest acid among the