Multiple Choice
Which metal sulfides can be precipitated from a solution that is 0.01 M in Mn2+,Zn2+,Pb2+ and Cu2+ and 0.10 M in H2S at a pH of 1.0? 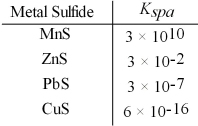
Definitions:
Related Questions
Q6: For the reaction N<sub>2</sub>(g)+ 2 O<sub>2</sub>(g)→ 2
Q20: If K<sub>c</sub> equals 0.11 at 25°C for
Q38: The following pictures represent mixtures of A<sub>2</sub>B<sub>4</sub>
Q44: If this reaction is endothermic,which picture (2)-(4)represents
Q93: What is the total volume of hydrogen
Q123: What is the best balanced chemical equation
Q145: What are the Br∅nsted-Lowry acids in the
Q172: What is the Al<sup>3+</sup>:Ag<sup>+</sup>concentration ratio in the
Q183: What is the pH of a solution
Q223: What is the geometric shape of the