The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 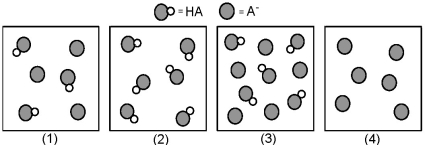
-Which solution has the largest percent dissociation of HA?
Definitions:
Methyl Propanoate
An ester formed from methanol and propanoic acid, often used as a solvent or in the synthesis of other compounds.
Methods
Systematic approaches or techniques used to accomplish a task, solve problems, or conduct research.
Major Product
In a given chemical reaction, the principal substance produced, distinguished by its predominance among other possible products.
Acetic Formic Anhydride
A mixed anhydride derived from acetic and formic acids, used in organic synthesis, particularly in acetylation reactions.
Q28: Which one of the following binary oxides
Q62: For which one of the following reactions
Q77: A reaction for which ΔH° = +
Q99: Write the equilibrium equation for the reverse
Q104: If ΔG° is negative for a reaction,<br>A)K
Q119: The equilibrium constant is equal to 5.00
Q132: When 50 mL of 0.10 M NH<sub>4</sub>Cl
Q143: Addition of 0.0125 mol HCl to 150
Q153: What is the equation relating the equilibrium
Q188: Normal rainfall has a concentration of OH<sup>-</sup>