The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 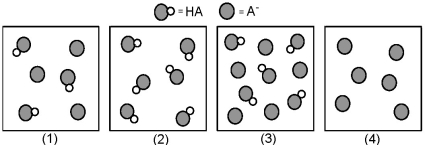
-Which solution has the greatest buffer capacity?
Definitions:
Strategic Alliance
A partnership formed to create competitive advantage on a worldwide basis.
Q25: How many mL of O<sub>2</sub> gas at
Q44: Methylamine CH<sub>3</sub>NH<sub>2</sub>,has a base dissociation constant of
Q70: Phosphorus pentachloride decomposes to phosphorus trichloride and
Q75: Write the equilibrium equation for the forward
Q81: What is the pH of a solution
Q95: At a certain temperature,K<sub>c</sub> equals 1.4 ×
Q98: What is the reduction half-reaction for the
Q129: Given the hypothetical reaction: 2 A(s)+ x
Q137: The Br∅nsted-Lowry acids in the chemical equation
Q162: How many liters of O<sub>2</sub> gas at