The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 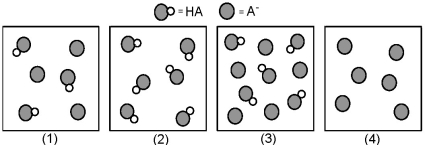
-Which solution has the greatest buffer capacity?
Definitions:
Homogenization
The process of making things uniform or similar, often seen in cultures losing distinct features due to globalization.
Regionalization
Is the division of the world into different and often competing economic, political, and cultural areas.
Sociologists
Scholars and specialists who study society and social behavior by examining the groups, cultures, organizations, social institutions, and processes that develop when people interact and work together.
Source
The origin or provider of information, materials, or evidence, used in various contexts including research, journalism, and supply chains.
Q10: Solid NaHCO<sub>3</sub> is heated to 90°C.At equilibrium
Q10: Given: Ag<sup>+</sup>(aq)+ e<sup>-</sup> → Ag(s)E° = +0.799
Q13: In the preparation of oxygen by the
Q120: Potassium hydrogen phthalate (molar mass = 204.2
Q144: Which is a net ionic equation for
Q155: A gaseous compound,C,undergoes catalytic decomposition at an
Q162: How many liters of O<sub>2</sub> gas at
Q171: Sulfurous acid,H<sub>2</sub>SO<sub>3</sub> has acid dissociation constants K<sub>a1</sub>
Q185: What is the pH of a solution
Q223: What is the geometric shape of the