The following pictures represent solutions that contain a weak acid HA (pKa = 5.0) and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 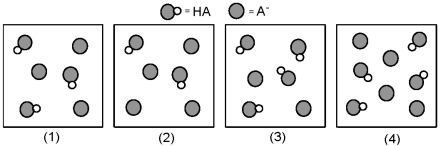
-For which of these solutions is pH = pKa?
Definitions:
Competencies
Skills and abilities that enable an individual to perform tasks successfully.
Diagnose Conflict
The process of identifying the source, nature, and scope of a disagreement or dispute.
Distributive Negotiations
A negotiation method that involves dividing a fixed amount of resources or assets, where any gain by one party is made at the expense of another.
Mutually Acceptable Solution
An outcome or resolution agreed upon by all parties involved, typically in a negotiation or dispute resolution context.
Q21: For a dead battery<br>A)E is negative and
Q27: A solution with a hydroxide ion concentration
Q30: What is the value of the equilibrium
Q32: For the reaction shown below,which change in
Q34: Which one of the following is not
Q60: What is the hydronium ion concentration of
Q69: Given that Cl<sub>2</sub>(g)+ 2 e<sup>-</sup> ?
Q86: Determine the acid dissociation constant for a
Q106: What are the signs (+ or -)of
Q164: Which acid of the following set has