The following pictures represent solutions at various points in the titration of a weak acid HA with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity) . 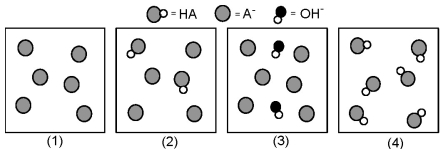
-Which picture represents the solution before the equivalence point?
Definitions:
Factory Supplies Expense
The cost associated with supplies used in the production process within a manufacturing facility.
Depreciation Expense-Factory
The portion of a factory's fixed assets' cost allocated as an expense during a fiscal period, due to wear and tear.
Permanent/Temporary
Refers to elements in financial or other records that either remain constant over time (permanent) or exist only for a specific period before being eliminated (temporary).
Financial Statement
A formal record of the financial activities and position of a business, individual, or other entity.
Q33: The brown color associated with photochemical smog
Q70: Classify each of the following processes as
Q110: Arrange the acids in order of increasing
Q112: Shown below are the reactions occurring in
Q121: Based on the half-reactions and their respective
Q131: What is the pH of a 0.100
Q139: What is the most soluble salt of
Q152: The chlor-alkali industry is based on the
Q170: A solution may contain the following ions
Q206: What is the pH of a 0.020