The following pictures represent solutions of CaCO3,which may also contain ions other than Ca2+ and CO32- which are not shown.Gray spheres represent Ca2+ ions and unshaded spheres represent CO32- ions. 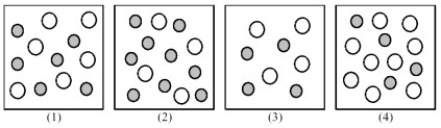
-If solution (1) is a saturated solution of CaCO3,which of solutions (1) -(4) represents the solution after a small amount of K2CO3 is added and equilibrium is restored?
Definitions:
Yield To Maturity
The total return expected on a bond if held until the end of its lifetime.
Yield To Maturity
The total expected return on a bond if it is held until the date it matures.
Q5: The reaction 2 H<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 H<sub>2</sub>O(g)is
Q8: For bromine,ΔH°vap = 30.91 kJ/mol and ΔS°vap
Q18: At a certain temperature the equilibrium constant,K<sub>c</sub>,equals
Q26: The enthalpy for the following reaction is
Q28: The entropy change associated with the expansion
Q59: Which of the following are unstable with
Q84: When 5.60 grams of anhydrous copper(II)sulfate is
Q104: Calculate the value of the reaction quotient,Q,for
Q140: A tablet containing 500.0 mg of aspirin
Q156: The neutralization constant K<sub>n</sub> for the neutralization