Calculate the standard free energy for the reaction given.
2 CH3OH(l) + 3 O2(g) → 2 CO2(g) + 4 H2O(l) 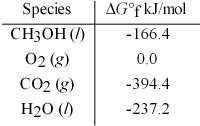
Definitions:
Lecture Material
The content provided or discussed by an instructor during a lecture, including theories, concepts, and key points.
Notes
Written reminders or brief records made to assist in remembering information or topics discussed.
Reading
The process of understanding and interpreting written symbols and texts.
Marked
Graded or evaluated, often used in the context of academic work or assessment to denote that it has been reviewed and given a score or feedback.
Q54: In which of the following reactions does
Q55: Which one of the following would be
Q81: What is the pH of a solution
Q84: When 5.60 grams of anhydrous copper(II)sulfate is
Q106: What are the common oxidation states of
Q115: The first step in the steam-hydrocarbon re-forming
Q138: Which of the following reactions are not
Q142: For which of the following will the
Q167: Which of the following chemical reagents is
Q209: A Br∅nsted-Lowry acid is best defined as