In Figure (1)below Oxygen Molecules,represented by Unshaded Spheres,and Chlorine Molecules,represented
In figure (1) below oxygen molecules,represented by unshaded spheres,and chlorine molecules,represented by shaded spheres,are in separate compartments.Figure (2) shows the equilibrium state of the system after the stopcock separating the two compartments is opened.Assuming the oxygen and the chlorine behave as ideal gases,what are the signs (+,-,or 0) of ΔH,ΔS,and ΔG for this process? 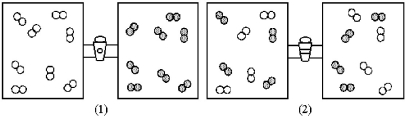
Definitions:
Considerable Emphasis
Significant importance or stress placed on a particular aspect or detail.
State
An organized political community under one government, recognized as sovereign or independent.
Inequality
A situation where resources, opportunities, and rights are distributed unevenly amongst individuals or groups in a society.
Regulate Inequality
Efforts or policies aimed at managing or reducing the disparities in wealth, resources, or opportunities among members of a society.
Q5: The reaction 2 H<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 H<sub>2</sub>O(g)is
Q9: What is the hydroxide ion concentration of
Q24: The balanced equation for the solubility equilibrium
Q72: Consider the galvanic cell,Pb(s)| Pb2+(aq)|| Cu<sup>2+</sup>(aq)| Cu(s).Which
Q80: What are the three major chemicals that
Q87: What is the charge,n,in the ion Si<sub>4</sub>O<sub>10</sub><sup>n</sup>
Q108: Which has the highest standard molar entropy
Q143: Which forward reaction is a nonspontaneous process?<br>A)the
Q176: Which of the following elements forms the
Q180: The strongest reducing agent in the group