The figure below represents the spontaneous reaction of H2 (shaded spheres) with O2 (unshaded spheres) to produce gaseous H2O. 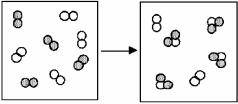
-What are the signs (+,or -) of ΔH,ΔS,and ΔG for this process?
Definitions:
Innate Immunity
The first line of defense in the immune system, consisting of physical, chemical, and cellular defenses against pathogens that are present from birth.
Multicellular Parasites
Multicellular parasites are organisms made up of many cells that live at the expense of a host organism, causing harm or disease.
Granules
Small particles or grains, often referring to structures within cells that store specific substances.
Cascading Reactions
A series of sequential chemical or biochemical reactions where the product of one reaction becomes the reactant of the next, often leading to complex outcomes.
Q32: Using the following standard reduction potentials<br>Fe<sup>3+</sup>(aq)+ e<sup>-</sup>
Q33: Which picture represents the system beyond the
Q44: Which element of group 4A has the
Q47: The figure above represents the nonspontaneous reaction
Q62: According to the third law of thermodynamics,<br>A)energy
Q63: Which acid has the lowest percent dissociation?<br>A)HX<br>B)HY<br>C)HZ<br>D)All
Q83: What is the pH of the solution
Q90: What is the shorthand notation that represents
Q111: The initial concentrations of Ag<sup>+</sup>(aq)and Cu<sup>2+</sup>(aq)are both
Q221: Dihydrogen phosphate H<sub>2</sub>PO<sub>4</sub><sup>-</sup>,has an acid dissociation constant