Consider the following gas-phase reaction of A2 (shaded spheres) and B2 (unshaded spheres) :
A2(g) + B2(g) ⇌ 2 AB(g) ΔG ° = +25 kJ 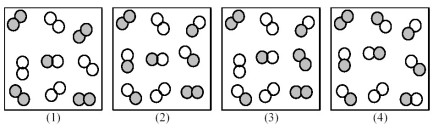
-Which of the above reaction mixtures has the least spontaneous forward reaction?
Definitions:
Senescence
The process of biological aging at the cellular level, marked by the gradual deterioration of function and increased risk of death.
Wrinkles
Lines, creases, or folds in the skin that primarily appear as people age due to loss of skin elasticity and moisture.
Hearing Aids
Electronic devices designed to improve the hearing of those with hearing loss by amplifying sound.
Severe Brain Loss
A significant reduction in brain tissue, which can result from various conditions such as Alzheimer's disease, trauma, or other neurodegenerative diseases, leading to cognitive decline and functional impairment.
Q3: Use Table 17.1 to calculate the standard
Q14: What is the molecular structure of phosphorous
Q58: What is the hydronium ion concentration of
Q64: For the galvanic cell shown above,in what
Q85: The gas OF<sub>2</sub> can be produced from
Q110: Arrange the acids in order of increasing
Q120: At 25°C,ΔG° = -198 kJ for the
Q141: Calculate the pH for an aqueous solution
Q148: What is the pH of a 0.020
Q184: A buffer solution is prepared by dissolving