Consider the following gas-phase reaction of A2 (shaded spheres) and B2 (unshaded spheres) :
A2(g) + B2(g) ⇌ 2 AB(g) ΔG ° = +25 kJ 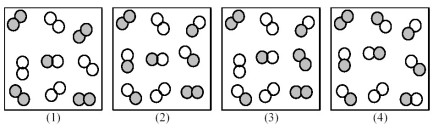
-Which of the above reaction mixtures is ΔG of reaction = ΔG °?
Definitions:
Consume
The action of using up goods or services, thus leading to a reduction in available quantity.
GDP
The entirety of value generated from goods and services within a country's borders throughout a specific duration sums up what is known as the Gross Domestic Product.
Government Purchases
Expenditures by government for goods and services that directly satisfy collective needs or affect the economy’s total demand.
Investment
Investment pertains to the allocation of resources, such as capital or time, with the expectation of generating an income or profit.
Q6: A solution with a hydroxide ion concentration
Q29: The signs of ΔG,ΔH,and ΔS at 25°C
Q67: An electron in an oxygen p orbital
Q75: Which is the lowest at 25°C?<br>A)ΔG°<sub>f</sub> for
Q102: For the evaporation of water during perspiration
Q110: ΔS° = -198.7 J/K for the reaction
Q121: For any thermodynamic function Y,ΔY° for a
Q125: What is the pH at the first
Q143: Identify the anode and cathode half-reactions and
Q180: What is the molar solubility of Mg(OH)<sub>2</sub>