Calculate the equilibrium constant,K,at 25°C for the galvanic cell reaction shown below: 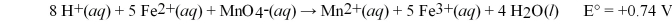
Definitions:
Holding Cost Rate
The percentage of the value of inventory that is held as a cost for storing, insuring, and managing goods over a certain period.
Chilliwack
A city in the province of British Columbia, Canada, known for its natural beauty and outdoor activities.
Holding Cost Rate
The percentage of the inventory value held that represents the cost to store, manage, and insure inventory over a certain period.
Montreal
A major city in Quebec, Canada, known for its cultural diversity, historical sites, and economic significance.
Q3: From the above models of hydrides,indicate the
Q12: Which statement is true?<br>A)The cathode is positive
Q30: Which oxoanion is the strongest acid?<br>A)oxoanion (1)<br>B)oxoanion
Q31: In which of the following solutions would
Q57: When P<sub>4</sub>O<sub>10</sub> is dissolved in water,the water
Q82: A galvanic cell consists of a Ni<sup>2+</sup>/
Q104: Of the elements indicated on the periodic
Q124: What is the relationship between ΔG,Q<sub>p</sub>,and K<sub>p</sub>
Q165: According to Table 17.1,which aqueous metal ion
Q171: At 25°C the elements indicated by the