Shown below is a galvanic cell with anode compartment b containing anode a and cathode compartment d containing cathode c.Electrons flow through wire f,ions flow through salt bridge e,and the cell potential is read using voltmeter g.
This galvanic cell uses the reaction: Cu(s) + 2 Ag+(aq) 2 Ag(s) + Cu2+(aq) . 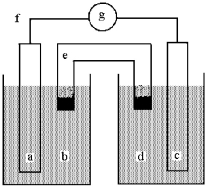
-NaNO3(aq) is employed in the salt bridge.Give the direction of electron flow and the direction of ion flow from the salt bridge.
Definitions:
Interim Statements
Financial reports covering a period of less than a full fiscal year, typically quarterly, intended to provide an update on an organization's financial position.
Financial Position
A summary of the resources, obligations, and worth of an entity at a specific point in time, usually reflected in a balance sheet.
Year-End Financial Statements
Reports prepared at the end of an accounting period, summarizing the financial status and operating results of a business.
Management
involves the activities of setting the strategy of an organization and coordinating the efforts of its employees or volunteers to achieve its objectives.
Q8: Calculate the value of the reaction quotient,Q,for
Q18: How many grams of KCl(s)are produced from
Q20: How many moles of electrons,n,are transferred in
Q89: What is one method of making "laughing
Q105: What is the oxidation number of oxygen
Q111: Calculate the molar solubility of thallium(I)chloride in
Q114: An element that forms a basic oxide
Q125: Which compound is not considered to be
Q143: What is responsible for the brownish colored
Q154: Calculate the pH of a 1.60 M