Shown below is a galvanic cell with anode compartment b containing anode a and cathode compartment d containing cathode c.Electrons flow through wire f,ions flow through salt bridge e,and the cell potential is read using voltmeter g.
This galvanic cell uses the reaction: Cu(s) + 2 Ag+(aq) 2 Ag(s) + Cu2+(aq) . 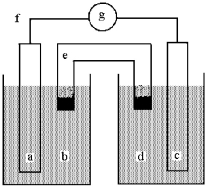
-The initial concentrations of Ag+(aq) and Cu2+(aq) are both 1.0 M.What will happen to the cell voltage if 1.0 M Cu(NO3) 2 is added to the compartment containing the 1.0 M Cu2+(aq) ? The cell voltage will
Definitions:
Receiving
The process of accepting delivery of goods or materials into a facility, checking them for accuracy against the purchase order, and inspecting for damage or defects.
Inspection
The process of examining materials, products, or systems to ensure they meet certain standards, regulations, or customer expectations.
Physical Handling
The process involving the manual or mechanical manipulation and movement of physical goods and materials.
Payment Approval
The process of verifying and authorizing the release of funds to settle a transaction or invoice.
Q20: The number of transition series is<br>A)one<br>B)two<br>C)four<br>D)seven
Q28: Which one of the following binary oxides
Q38: How many grams of chromium metal are
Q54: What is the structure of white phosphorus?<br>A)cage
Q77: Consider the following standard reduction potentials,<br>Al<sup>3+</sup>(aq)+ 3
Q79: Which of the following elements has the
Q84: If solution (1)is a saturated solution of
Q146: Which one of the following elements can
Q156: What are the allotropes of oxygen?<br>A)O and
Q161: How many valence electrons does the element