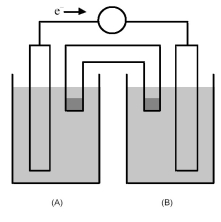
-The cell reaction 2 Fe3+(aq) + Zn(s) → Zn2+(aq) + 2 Fe2+(aq) occurs in the galvanic cell shown above.Which would be the most appropriate choices for the solid electrode in half-cell (A) and in half-cell (B) ?
Definitions:
Group Harmony
The state of agreement, peace, and unity within a group, where members often prioritize collective well-being over individual desires.
Happiness Levels
A measure of well-being or joy in individuals or groups, often influenced by various personal, social, and environmental factors.
Overestimate
To assess something as being larger, better, or more important than it actually is.
Underestimate
To judge something below its actual value, size, or importance.
Q14: The pH of a 0.150 M formic
Q33: Which picture represents the system beyond the
Q59: Which of these molecules is most likely
Q67: Bromothymol blue indicator changes color from yellow
Q69: What is the molar solubility of AgCl
Q97: Which element of group 6A has the
Q99: During an electrochemical reaction,electrons move through the
Q104: Electrical conductivity in graphite is maximized _.<br>A)parallel
Q130: What is the molar solubility of CaF<sub>2</sub>
Q202: What statement is inconsistent about carbon monoxide?<br>A)It