Multiple Choice
Consider the following galvanic cell. 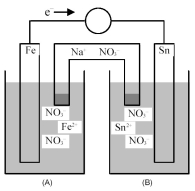
-What is the balanced equation for the cell reaction?
Definitions:
Related Questions
Q10: Given: Ag<sup>+</sup>(aq)+ e<sup>-</sup> → Ag(s)E° = +0.799
Q12: How many liters of oxygen gas can
Q12: Which statement is true?<br>A)The cathode is positive
Q12: By what factor does the entropy increase
Q61: What is the approximate pH at the
Q69: Which element indicated on the above periodic
Q72: What is the most soluble salt of
Q77: What oxidation state(s)is(are)exhibited by all first row
Q98: Standard molar entropies,S°,in J/Kmol,are given below each
Q202: What statement is inconsistent about carbon monoxide?<br>A)It