Shown below is a galvanic cell with anode compartment b containing anode a and cathode compartment d containing cathode c.Electrons flow through wire f,ions flow through salt bridge e,and the cell potential is read using voltmeter g.
This galvanic cell uses the reaction: Cu(s) + 2 Ag+(aq) 2 Ag(s) + Cu2+(aq) . 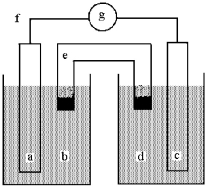
-The initial concentrations of Ag+(aq) and Cu2+(aq) are both 1.0 M.What will happen to the cell voltage if 5.0 M Cu(NO3) 2 is added to the compartment containing the 1.0 M Cu2+(aq) ? The cell voltage will
Definitions:
Slave States
States in the U.S. before 1865 where the institution of slavery was legally permitted.
Union Army
The land force that fought for the Northern states during the American Civil War, opposing the Confederacy and fighting to preserve the Union and end slavery.
Steamer Planter
A Confederate steamship hijacked in 1862 by Robert Smalls, an enslaved African American who then sailed it to freedom and surrendered it to the Union.
Robert Smalls
An African American who escaped slavery to become a Union naval hero and later a US Congressman.
Q9: Among the main-group elements the number of
Q28: The entropy change associated with the expansion
Q34: Which oxide is the most basic?<br>A)A<br>B)B<br>C)C<br>D)D
Q36: What is the oxidation number of carbon
Q41: If solution (1)is a saturated solution of
Q57: What is the pH of a solution
Q88: Commercially oxygen is usually obtained by<br>A)decomposition of
Q96: Calculate the standard free energy change at
Q115: The first step in the steam-hydrocarbon re-forming
Q139: What is the most soluble salt of