What is the total volume of the mixture of hydrogen gas and oxygen gas can be obtained from the electrolysis of 90.0 grams of water at 25.0°C and 1.00 atm pressure according to the chemical equation shown below?
2 H2O(l) 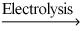 2 H2(g) + O2(g)
2 H2(g) + O2(g)
Definitions:
Q7: How many liters of O<sub>2</sub> gas at
Q16: Which is classified as an amphoteric binary
Q32: Using the following standard reduction potentials<br>Fe<sup>3+</sup>(aq)+ e<sup>-</sup>
Q42: The most acidic oxides of the group
Q64: Which of the following metal hydroxides are
Q83: The elements indicated by the shaded area
Q92: Which of the following processes is spontaneous?<br>A)a
Q105: What is the oxidation number of oxygen
Q129: For a galvanic cell that uses the
Q181: What statement is not consistent with the