Look at the location of elements A,B,C,and D in the following periodic table.Consider the oxides that form when the elements A-D are in their highest oxidation states. 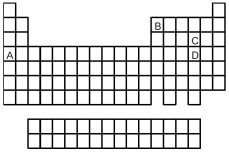
-Which oxide is the most basic?
Definitions:
Articles of Incorporation
Legal documents filed with a governmental body to legally document the creation of a corporation.
Bylaws
The set of rules and regulations that govern the internal management and structure of a corporation or organization.
Shareholder Agreement
A contract among a company's shareholders detailing the rights and obligations, often including aspects like voting rights and share sales.
MBCA
Stands for the Model Business Corporation Act, a model set of laws prepared by the Committee on Corporate Laws of the American Bar Association to guide states in the development of their corporate statutes.
Q3: Use Table 17.1 to calculate the standard
Q21: Which oxide has the lowest melting point?<br>A)A<br>B)B<br>C)C<br>D)D
Q65: The binary hydride shown in picture (3)is
Q97: Calculate the cell potential E at 25°C
Q105: The standard molar entropy for Br<sub>2</sub>(g)is 245.46
Q108: Which has the highest standard molar entropy
Q137: What is the chemical formula for the
Q156: What are the allotropes of oxygen?<br>A)O and
Q163: CaF<sub>2</sub> has K<sub>sp</sub> = 3.5 × 10<sup>-11</sup>.If
Q163: Which group 6A element is naturally radioactive?<br>A)S<br>B)Se<br>C)Te<br>D)Po