Look at the location of elements A,B,C,and D in the following periodic table.Consider the oxides that form when the elements A-D are in their highest oxidation states. 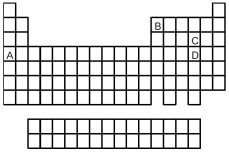
-Which oxide has the highest melting point?
Definitions:
Traditional Antipsychotic Drugs
Medications primarily used to manage psychosis, especially in schizophrenia and bipolar disorder, characterized by their dopamine antagonist properties.
Spastic Movements
Involuntary muscle contractions that are often sudden and uncontrolled, typically resulting from neurological or muscular disorders.
Antipsychotic Drugs
Medications used to treat psychiatric conditions by managing symptoms such as delusions, hallucinations, and disordered thinking.
Psychotic Patients
Individuals diagnosed with psychiatric disorders that significantly interfere with cognition and perception, affecting their ability to recognize reality.
Q1: How would one classify a germanium crystal
Q9: The nickel-cadmium battery cell has a standard
Q22: If the concentrations of Ag<sup>+</sup>(aq)and Cu<sup>2+</sup>(aq)are varied
Q67: Which one of the following is a
Q99: The number of unpaired electrons in tetrahedral
Q117: Vanadium ore contains two isotopes of vanadium,<sup>50</sup>V
Q122: Which of the following statements concerning a
Q127: Which oxoanion is the weakest base?<br>A)oxoanion (1)<br>B)oxoanion
Q130: Which of the Co(III)complexes above are optically
Q177: What is the ground-state electron configuration for