Look at the location of elements A,B,C,and D in the following periodic table.Consider the oxides that form when the elements A-D are in their highest oxidation states. 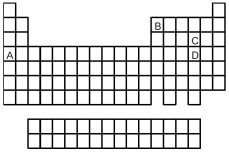
-Which oxide is the most covalent?
Definitions:
Capital Investment
Funds invested in a business with the expectation of future benefits, such as acquiring new equipment, buildings, or other resources to generate income.
Net Present Value
A method used to evaluate the profitability of an investment by calculating the difference between the present value of cash inflows and outflows over a period of time.
Capital Investment
Funds spent by a company to acquire or upgrade physical assets such as property, industrial buildings or equipment to increase operational efficiency.
Future Net Cash Flows
The estimated total cash income minus the total cash expenses expected over a future period.
Q77: The neutralization constant K<sub>n</sub> for the neutralization
Q90: The binary hydride shown in picture (2)is
Q95: What is the chemical equation for the
Q122: Which hydride dissolves in water to form
Q124: What is the relationship between ΔG,Q<sub>p</sub>,and K<sub>p</sub>
Q136: For the hypothetical reaction A + B<sup>x</sup>
Q147: Which element of group 6A has the
Q159: Which are trans-isomers?<br>A)isomers (1)and (2)<br>B)isomers (1)and (3)<br>C)isomers
Q169: Which of the following can function as
Q175: Which group 3A element exhibits the most