Look at the location of elements A,B,C,and D in the following periodic table.Consider the oxides that form when the elements A-D are in their highest oxidation states. 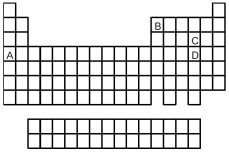
-Which oxide is a solid with an infinitely extended three-dimensional crystal structure?
Definitions:
Products and Services List
A comprehensive categorization of the goods sold and services offered by a business, often used for inventory management and sales record.
3-Way Match
A process in accounting used to compare the purchase order, the receiving report, and the supplier's invoice before making a payment.
Internal Control
Measures and protocols established within an organization to safeguard its assets, enhance the reliability of its accounting records, and increase efficiency in operations.
Bundle
A group of products or services sold together, often at a reduced price compared to purchasing each item individually.
Q20: Superoxide ion,O<sub>2</sub><sup>-</sup>,peroxide ion,O<sub>2</sub><sup>2-</sup>,and oxide ion,O<sup>2-</sup>,contain _,_,and _
Q28: Which one of the following binary oxides
Q47: Using Table 17.1,find E° for 2 H<sub>2</sub>O(l)→
Q56: Which are cis-isomers?<br>A)isomers (1)and (2)<br>B)isomers (1)and (3)<br>C)isomers
Q62: What is least easily oxidized?<br>A)Al<br>B)Fe<br>C)Mg<br>D)Zn
Q85: Which statement is true about the formation
Q96: What is the range of oxidation states
Q124: What is the relationship between ΔG,Q<sub>p</sub>,and K<sub>p</sub>
Q135: Using the above schematic of an artist's
Q140: Formic acid (HCO<sub>2</sub>H,K<sub>a</sub> = 1.8 × 10<sup>-4</sup>)is