Given the thermochemical equation
2Al(s) + 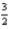
O2(g) → Al2O3(s) ; ΔH = -1676 kJ
Find ΔH for the following reaction.
2Al2O3(s) → 4Al(s) + 3O2(g)
Definitions:
Association
A connection or relationship between two or more variables, where changes in one variable correspond to changes in another.
Mean
In statistics, it is the arithmetic average of a set of numbers, calculated by adding them all up and dividing by the number of numbers.
Exceptionally High
Significantly surpassing the usual or average level, often in a way that is rare or unparalleled.
Participant Observation
A method used in social science research involving the immersion of the researcher in the environment of their subjects to observe and engage in their activities to gain a deeper understanding of their culture or behavior.
Q3: A crystal of the mineral troegerite,(UO<sub>2</sub>)<sub>3</sub>(AsO<sub>4</sub>)<sub>2</sub> •
Q5: The analysis of an organic compound showed
Q11: Which of the following molecules contains the
Q23: Which of the following Lewis formulas is
Q28: The Pauli exclusion principle states that<br>A)the wavelength
Q62: Which of the following is a weak
Q66: If four orbitals on one atom overlap
Q81: What is the standard enthalpy of formation
Q112: The reaction of H<sub>2</sub>SO<sub>4</sub> with NaOH is
Q148: Which of the following reactions best describes