What is the quantity of heat evolved at constant pressure when 30.5 g H2O(l) is formed from the combustion of H2(g) and O2(g) ?
H2(g) + 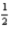
O2(g) → H2O(l) ; ΔH° = -285.8 kJ
Definitions:
Increase in IQ
A measurable improvement in intelligence quotient, indicating enhanced cognitive abilities.
Middle-Class Parents
Middle-class parents typically refer to parents from the socio-economic class characterized by moderate income levels, often associated with specific parenting styles and educational aspirations for their children.
Understaffed Orphanage
A childcare facility lacking sufficient staff to adequately care for the number of children housed.
Non-Twin Siblings
Brothers or sisters who are born from the same parents but at different times, not as part of a multiple birth.
Q5: What precipitate forms when aqueous solutions of
Q6: If q = -96 kJ for a
Q22: What is the percent by mass nitrogen
Q37: All of the following species are isoelectronic
Q51: A precipitate is expected when an aqueous
Q54: Which molecule or ion is not planar?<br>A)H<sub>2</sub>CO<br>B)NO<sub>2</sub><sup>-</sup><br>C)C<sub>2</sub>F<sub>4</sub><br>D)H<sub>2</sub>CCO<br>E)PO<sub>4</sub><sup>3-</sup>
Q82: What is the electron geometry (or electron
Q97: The molar mass of an unknown gas
Q101: The following equation represents the partial combustion
Q154: What is the molarity of hydrochloric acid