What quantity,in moles,of oxygen is consumed when 717.4 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ?
H2(g) + 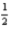
O2(g) → H2O(l) ; ΔH° = -285.8 kJ
Definitions:
Confirmation Bias
The inclination to look for, interpret, prioritize, and remember information in a manner that validates one's existing beliefs or assumptions.
Availability Heuristic
A fast-track cognitive process that uses the earliest examples coming to mind for a person when evaluating a unique topic, concept, practice, or choice.
Gambler's Fallacy
The inaccurate belief that when something is happening more frequently than is typical during a particular time, it will subsequently happen less frequently, or the other way around.
Maladaptiveness
A trait or behavior that is counterproductive to an individual's well-being or ability to meet demands of the environment.
Q2: An atom of which of the following
Q33: Which of the following compounds has the
Q38: How many p orbitals are in the
Q50: What mass of oxalic acid dihydrate,H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> ·
Q62: What is the mass in grams of
Q93: How much heat is released at constant
Q93: Which of the following forms the most
Q124: An atom of which of the following
Q127: Which of the following reactions best describes
Q137: A sample containing 0.400 mol of a