How much heat is evolved upon the complete oxidation of 6 g of aluminum at 25°C and 1 atm pressure? ( 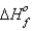 for Al2O3 is -1676 kJ/mol.)
for Al2O3 is -1676 kJ/mol.)
4Al(s) + 3O2(g) → 2Al2O3(s)
Definitions:
Net Income
A company’s final income after deducting all expenses and taxes from its gross revenue.
Domestic Corporations
Corporations that are incorporated and operate within the legal boundaries of a particular country, subject to that country's laws and regulations.
Deferred Income Tax Liability
A tax obligation due in the future for income already earned and recognized for accounting purposes.
Operating Income
Income generated from the regular operating activities of a business, excluding revenues and expenses from non-operating sources like investments.
Q1: What is the standard enthalpy change for
Q6: If q = -96 kJ for a
Q12: A photon of red light has a
Q27: Which of the following compounds contains the
Q39: A particular gas exerts a pressure of
Q68: Ammonia can be made by reaction of
Q71: The empirical formula of butadiene is C<sub>2</sub>H<sub>3</sub>.An
Q72: When the following equation is balanced with
Q87: A fixed amount of gas in a
Q109: Which of the following chemical equations is