Given the following data,calculate the standard enthalpy of reaction for the conversion of buckminsterfullerene (C60) into diamond: 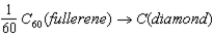 C(graphite) → C(diamond) ; ΔH° = +1.897 kJ
C(graphite) → C(diamond) ; ΔH° = +1.897 kJ
60C(graphite) → C60(fullerene) ; ΔH° = +2193 kJ
Definitions:
Authority
The power or right, usually backed by law, to give orders, make decisions, and enforce obedience.
Banquet Cancellation
The act of calling off or terminating a planned banquet event, typically due to unforeseen circumstances or breach of contract.
Accounting Firm
A business that provides services related to financial record keeping, audit, and taxation.
Seminar
An instructional event typically held to discuss or explore specific subjects or to provide training on a particular topic.
Q16: Which of the following statements is incorrect?<br>A)Stern
Q18: Which of the following electron configurations corresponds
Q63: Which of the following covalent molecules does
Q84: What is the standard enthalpy of formation
Q86: The ground-state valence-shell configuration of a particular
Q95: Which of the following reactions best describes
Q103: In the Born-Haber cycle for NaCl(s),which of
Q119: Which of the following statements concerning lattice
Q125: Which of the following chemical equations best
Q156: What mass of H<sub>3</sub>PO<sub>4</sub> (98.0 g/mol)is present