From the following information,determine the enthalpy of formation of C2H4(g) . 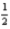 C2H4(g) → C(s) + H2(g) ; ΔH = -26.2 kJ
C2H4(g) → C(s) + H2(g) ; ΔH = -26.2 kJ
Definitions:
Competitive Firm
A business that competes in a market where it has some influence on the prices of its products or services but faces competition from other firms.
Total Economic
A term likely intending to refer to 'total economic value,' which encompasses all direct and indirect values of a resource or entity.
ATC Curves
Graphs that illustrate the average total cost of production at different levels of output.
Economic Profits
The difference between total revenue and total costs, including both explicit and implicit costs, indicating the profitability of a firm beyond normal profit levels.
Q1: Based on the photoelectric effect,Einstein proposed the
Q3: All the following statements about the quantum
Q13: Which of the following species would you
Q15: Which of the following statements concerning the
Q26: What volume of ammonia gas,measured at 547.9
Q35: Whose postulates account for the line spectrum
Q36: What is the net ionic equation for
Q62: A certain solid substance that is very
Q74: Which set of ions are isoelectronic in
Q80: Assume 12,500 J of energy is added