Given the thermochemical equation
2Al(s) + 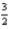
O2(g) → Al2O3(s) ; ΔH = -1676 kJ
Find ΔH for the following reaction.
2Al2O3(s) → 4Al(s) + 3O2(g)
Definitions:
Nara
A city in Japan known for its historic temples and artwork from the Japanese Nara period (710-794).
Muromachi
A period in Japanese history (1336-1573) characterized by the cultural achievements and military conflicts during the rule of the Ashikaga Shogunate.
Edo
The historical name for Tokyo during the period from 1603 to 1868, when it was the seat of the Tokugawa shogunate in Japan.
Hokusai
A Japanese artist, ukiyo-e painter, and printmaker of the Edo period, known for the iconic woodblock print series "Thirty-Six Views of Mount Fuji".
Q23: What is the molecular geometry around an
Q24: Which molecule or ion is nonlinear?<br>A)CO<sub>2</sub><br>B)NF<sub>2</sub><sup>-</sup><br>C)OCN<sup>-</sup><br>D)NO<sub>2</sub><sup>+</sup><br>E)HCCH
Q30: Nitric oxide,NO,is made from the oxidation of
Q54: Which of the following statements is incorrect
Q64: Which molecule or ion does not have
Q68: In order to dilute 71.1 mL of
Q72: One step in the isolation of pure
Q106: The formation of which monatomic ion of
Q114: In 1928,1.0 g of rhenium,Re,was isolated from
Q151: The sum of all the oxidation numbers