Given: 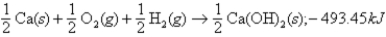 what is ΔH for the following thermochemical equation?
what is ΔH for the following thermochemical equation? 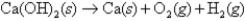
Definitions:
Hourly Worker
An employee whose pay is based on the number of hours worked rather than receiving a fixed salary.
Shop Foreman
A supervisory role within a manufacturing or industrial site, overseeing the work of employees and ensuring the efficiency and quality of production.
Work-In-Process Inventory
Goods that are partially completed in the manufacturing process but not yet ready for sale.
Cost Of Goods Sold
In a perpetual inventory system, an account that records the cost of merchandise inventory used to make the sale. Also, it is the total cost of the goods which were sold to customers.
Q10: Which molecule is polar?<br>A)C<sub>2</sub>H<sub>4</sub><br>B)CS<sub>2</sub><br>C)C<sub>6</sub>H<sub>6</sub><br>D)SO<sub>2</sub><br>E)CF<sub>4</sub>
Q13: What is the value of the principal
Q15: In the Lewis formula for hydrazinium ion,N<sub>2</sub>H<sub>5</sub><sup>+</sup>,the
Q26: Rank the following ions in order of
Q49: The following gases are stored in identical
Q53: Besides its ability to function as an
Q60: Consider the following changes at constant temperature
Q70: Which of the following is a weak
Q73: How much heat is gained by copper
Q79: A sample of 496 g of white