Which of the following statements is false concerning the reaction of hydrogen gas and oxygen gas given below?
H2(g) + 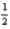
O2(g) → H2O(l) ; ΔH = -285.8 kJ
Definitions:
Advanced Paralegal Certification
A qualification indicating a higher level of expertise in paralegal studies, usually obtained through further education and testing.
Legal Specialty
An area of law practice that focuses on a particular field or subject of law.
General Legal Competence
The basic ability to understand, participate in, and engage with legal processes.
CLA Exam
A certification test for legal assistants or paralegals to demonstrate their proficiency and professional competence.
Q15: Which of the following is a representation
Q17: What is the molecular geometry around an
Q26: Rank the following ions in order of
Q28: What is the net ionic equation for
Q35: Which of the following electron configurations is
Q38: Which of the following electron configurations represents
Q63: Consider the following reaction:<br>2A + B →
Q111: In 0.266 mol of trimellitic acid,C<sub>6</sub>H<sub>3</sub>(COOH)<sub>3</sub>,there are<br>A)1.60
Q114: If more ice is added to an
Q139: Which is a reasonable mass corresponding to