What is the quantity of heat evolved at constant pressure when 30.5 g H2O(l) is formed from the combustion of H2(g) and O2(g) ?
H2(g) + 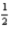
O2(g) → H2O(l) ; ΔH° = -285.8 kJ
Definitions:
Particular Activity
A specific action or endeavor undertaken by individuals or groups.
State of Mind
A person’s emotional and psychological condition at a particular time.
Supererogatory Actions
Actions that go beyond what is morally required or expected, typically considered voluntary and commendable but not obligatory.
Kant's Categorical Imperatives
Fundamental principles in Immanuel Kant's ethical theory that command us to act in a universally acceptable way, independent of our desires.
Q28: What is the standard enthalpy of formation
Q33: Which element forms the most acidic oxide?<br>A)B<br>B)Tl<br>C)Al<br>D)In<br>E)Ga
Q35: Which molecule or ion has a trigonal
Q38: The volume of 1 mol of nitrogen<br>A)is
Q47: Which of the following is a nonelectrolyte
Q52: In the following reaction,<br>2H<sub>2</sub>O<sub>2</sub>(l)→ 2H<sub>2</sub>O(l)+ O<sub>2</sub>(g)<br>Hydrogen peroxide
Q62: A fixed amount of gas in a
Q83: Which of the following conversions is not
Q91: The quantum numbers of an atom's highest-energy
Q111: For the reaction that occurs in a