What quantity,in moles,of oxygen is consumed when 717.4 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ?
H2(g) + 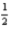
O2(g) → H2O(l) ; ΔH° = -285.8 kJ
Definitions:
Q2: An atom of which of the following
Q8: Which of the following processes will result
Q21: 2Al(s)+ 6HCl(aq)→ 2AlCl<sub>3</sub>(aq)+ 3H<sub>2</sub>(g)<br>According to the equation
Q31: One step in the isolation of pure
Q50: The energy that must be added to
Q71: Which molecule or ion is nonlinear?<br>A)N<sub>2</sub>O<br>B)O<sub>3</sub><br>C)OCN<sup>-</sup><br>D)NO<sub>2</sub><sup>+</sup><br>E)CS<sub>2</sub>
Q73: In a volumetric analysis experiment,a solution of
Q89: The Lewis structure for each of the
Q127: Which of the following reactions best describes
Q159: What is the net ionic equation for