How much heat is evolved upon the complete oxidation of 6 g of aluminum at 25°C and 1 atm pressure? ( 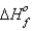 for Al2O3 is -1676 kJ/mol.)
for Al2O3 is -1676 kJ/mol.)
4Al(s) + 3O2(g) → 2Al2O3(s)
Definitions:
Ionic Compound
A substance made up of ions that are bonded together by the force of electrostatic attraction, known as ionic bonding.
Thallium(I) Nitrate
A chemical compound with the formula TlNO3, used in the manufacture of special glasses and in chemical synthesis.
Alkali Metals
A group of elements in the periodic table known for their high reactivity and presence of a single electron in their outermost shell.
Inner Transition Metals
Elements that include the lanthanides and actinides, found in the f-block of the periodic table, known for their complex electron configurations.
Q2: A 23.6 -L sample of nitrogen at
Q9: The balanced equation for the combustion of
Q11: Which of the following relates the rate
Q34: A 1.67-g sample of solid silver reacted
Q52: The enthalpy of fusion of aluminum is
Q59: What is the total number of subshells
Q103: Which of the following is an endothermic
Q111: What is the change in enthalpy at
Q114: In a certain experiment,0.7000 mol of hydrogen
Q150: Each of the following containers illustrates a