Multiple Choice
Given the following thermochemical data at 25°C and 1 atm pressure, 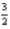 O2(g) + 2B(s) → B2O3(s) ; ΔH° = -1264 kJ
O2(g) + 2B(s) → B2O3(s) ; ΔH° = -1264 kJ
O3(g) + 2B(s) → B2O3(s) ; ΔH° = -1406 kJ
Determine ΔH° for the following reaction at 25°C and 1 atm pressure.
3O2(g) → 2O3(g)
Acknowledge the historical development of consumer credit.
Understand the sources and reliability of data used by credit bureaus.
Recognize the legal protections for consumers in the credit industry.
Identify the factors affecting the debt-to-equity ratio and its implications.
Definitions:
Related Questions
Q15: The skydiver plunging to earth possesses what
Q17: A 2.00-L glass soda bottle filled only
Q33: The frequency of a radio emission is
Q36: The contribution for which de Broglie is
Q40: How many molecules are there in 1.54
Q42: Which of the following orbital diagrams represents
Q47: Which of the following is a nonelectrolyte
Q83: Which conditions of P,T,and n,respectively,are most ideal?<br>A)low
Q105: How many moles of sulfate ions are
Q121: Which of the following is a strong