From the following information,determine the enthalpy of formation of C2H4(g) . 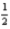 C2H4(g) → C(s) + H2(g) ; ΔH = -26.2 kJ
C2H4(g) → C(s) + H2(g) ; ΔH = -26.2 kJ
Definitions:
Field Diameter
The apparent size of an area visible through the microscope lens, often measured in millimeters or micrometers.
Millimeters
A unit of length in the metric system, equal to one thousandth of a meter.
Iris Diaphragm
A thin, adjustable circular structure in optical devices or the eye that controls the amount of light passing through by changing its size.
Condenser
A device used to condense a gas or vapor into a liquid.
Q7: Who postulated that energy is radiated only
Q12: An excess of sodium hydroxide is treated
Q17: A 2.00-L glass soda bottle filled only
Q18: What is the name for the following
Q20: The reaction of iron with hydrochloric acid
Q55: Identify the spectator ions in the following
Q66: The branch of physics that mathematically describes
Q75: Calculate the number of moles of O<sub>2</sub>
Q89: H<sub>2</sub> and F<sub>2</sub> react according to the
Q106: A 4.215 g sample of a compound