Consider the following energy-level diagram for a particular electron in an atom. 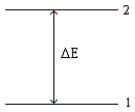 Based on this diagram,which of the following statements is incorrect?
Based on this diagram,which of the following statements is incorrect?
Definitions:
Agape Style
A selfless, sacrificial, unconditional love, often discussed in religious or philosophical contexts.
Suffer
To experience or undergo pain, distress, or hardship, which can be physical, emotional, financial, or of any other nature.
Survival of Species
The continued existence of a species through reproduction and adaptation to environmental changes, ensuring it does not become extinct.
Lee
A common surname that can have different origins, including English, Chinese, and Korean, often denoting a family lineage or heritage.
Q6: The following equation represents the oxidation of
Q37: The strongest intermolecular forces present in a
Q54: Which of the following species is isoelectronic
Q56: The square of the wave function,ψ<sup>2</sup>,of an
Q57: What is ΔH° for the following reaction?<br>2C<sub>2</sub>H<sub>2</sub>(g)+
Q88: The following species, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="The following
Q93: In which of the following species is
Q110: The molecules in a sample of solid
Q117: Which of the following is not a
Q151: The sum of all the oxidation numbers